The original article appeared on 3D Printing Industry and is slightly altered for our website. Netherlands based independent photopolymer 3Dprinting resin manufacturer Liqcreate launched Liqcreate Bio-Med Clear, the newest addition to their growing 3D-printing photopolymer resin portfolio. Bio-Med Clear is a biocompatible 3D printing resin, ideal for applications that require non-cytotoxic, non-sensitizing and non-irritating features.
Ruben Bosch, Marketing Sales and Executive at Liqcreate, states that “Liqcreate Bio-Med Clear is designed for a wide range of applications that require biocompatibility. This could range from skin contact applications to medical equipment that requires sterilization to be used in sterile environments.”
This rigid and clear photopolymer 3D-printing resin can be processed on most resin based 3D printers. Bio-Med Clear is compatible with Digital Light Processing (DLP), Liquid Crystal Display (LCD), and laser based 3D printing systems operating in the range 385nm to 420nm. Professional 3D-printers like the Asiga & atum3D, as well as entry level models from Phrozen, Elegoo, Creality & Anycubic, are compatible with this biocompatible resin. Find out if 3D-printing parameters for your 3D-printer are available here.
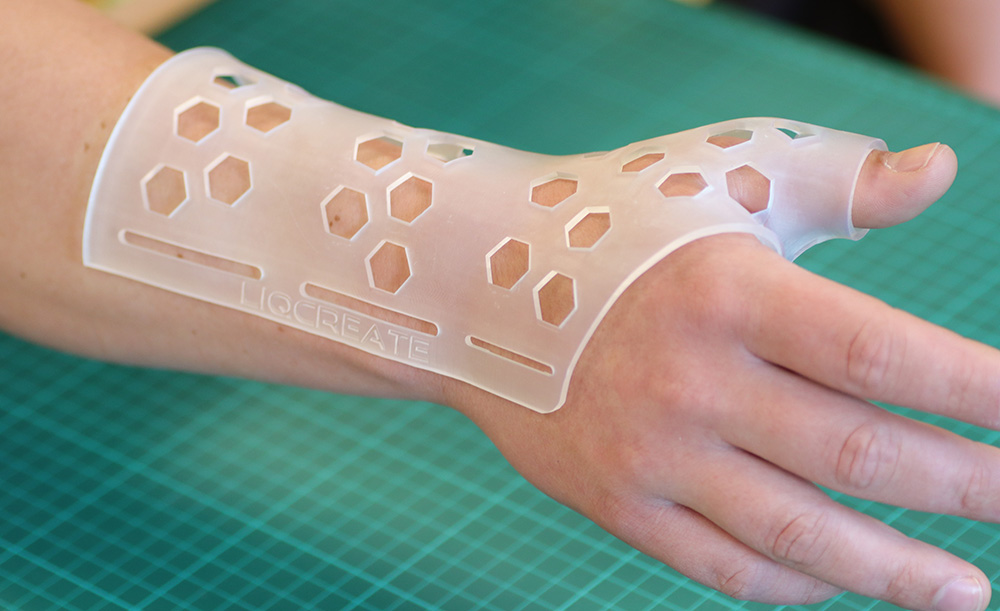
Example of an arm brace 3D-printed using Liqcreate Bio-Med Clear 3D resin.
Liqcreate Bio-Med Clear 3D printed parts can pass the following biocompatibility tests:
- – Cytotoxicity ISO 10993-5:2009
- – Sensitization ISO 10993-10:2021
- – Irritation ISO 10993-23:2021
As with all biocompatible materials, it is important to follow Liqcreate’s post-processing guidelines. These guidelines can be found on the on the page here. Besides, 3D-printed objects made with Bio-Med Clear can be disinfected with commonly used disinfectants and sterilized by steam sterilization using an autoclave. Defaukt steam sterilization programs have been validated at 121°C / 250 °F and at 134°C / 273°F for this biocompatible resin.
“Liqcreate Bio-Med Clear is designed to withstand elevated temperatures during steam sterilization, which does not influence final properties and part performance,” comments Jerzy Hul, Chief Scientific Officer at Liqcreate.
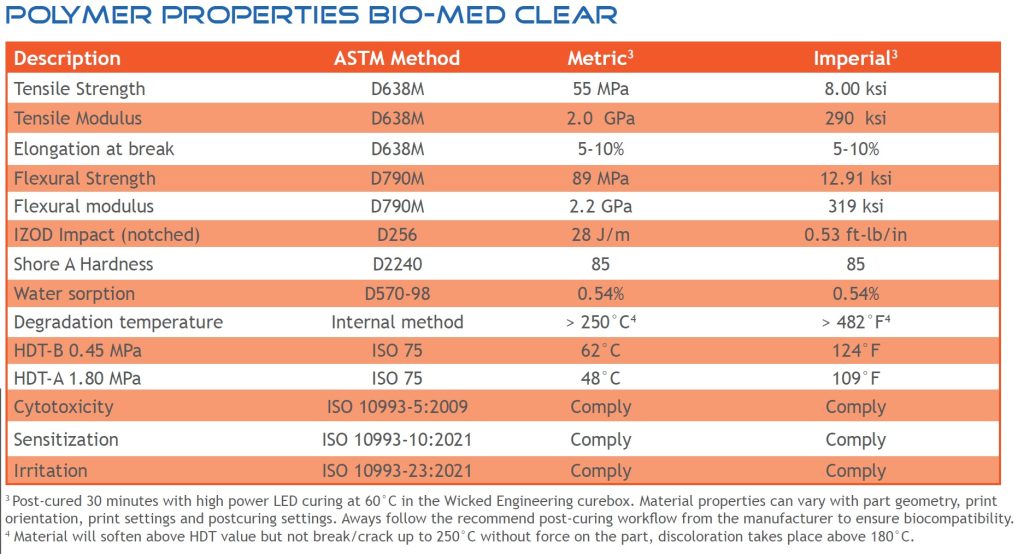
Liqcreate Bio-Med Clear polymer properties of biocompatible 3D-printing resin
Liqcreate Bio-Med Clear is perfect for applications that require biocompatible properties like: non-cytotoxic, non-sensitisation, and non-irritation. The below table provides information regarding the mechanical properties plastic parts made withLiqcreate Bio-Med Clear resin after post-curing.
The processing workflow of Liqcreate Bio-Med Clear, a biocompatible 3D-printing resin.
Iit is important for users to follow Liqcreate’s validated workflow instructions to achieve the previously shown properties and to ensure biocompatibility. Prior to printing, users must shake the resin bottle for 2 minutes and ensure that parts are 3D printed in an environment between 20-25°C / 68-77°F. Validated 3D printer settings for the 3D printing process can be found in this webpage.
When it comes to the wash and cure process, users should remove the parts from the build platform. Then followed by removing of the support structures. Next, it is recommended to wash the 3D printed parts for 2 minutes in IPA or Ethanol in an ultrasonic cleaner. These parts should then be washed for a second time in fresh IPA or Ethanol for another 2 minutes. The parts should be left to dry under ambient conditions after washing for at least 60 minutes. Last step of post-processing is to UV-cure the parts for 30 minutes at 60℃ in the Wicked Engineering Curebox.
Liqcreate advises that, when utilizing the Bio-Med Clear resin to produce regulated medical devices, customers should contact a Liqcreate representative to discuss the application before use.

Liqcreate Bio-Med Clear properties after autoclave or steam sterilization
In the table below the mechanical properties according to ASTM D790 and ISO 20795-2 are measured before and after steam sterilization. Although there are slight changes during steam sterilization, these are fairly small and do not alter the parts function.
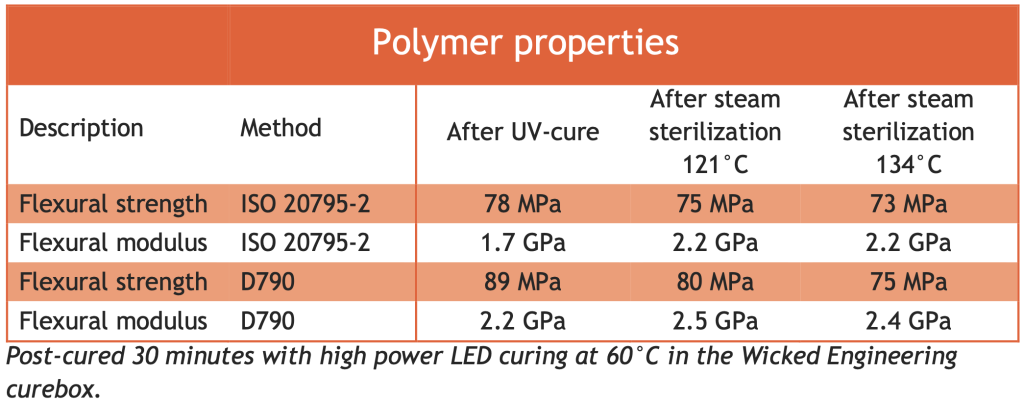
Comparison of properties after autoclave / steam sterilization of Liqcreate Bio-Med Clear, biocompatible 3D-print resin.
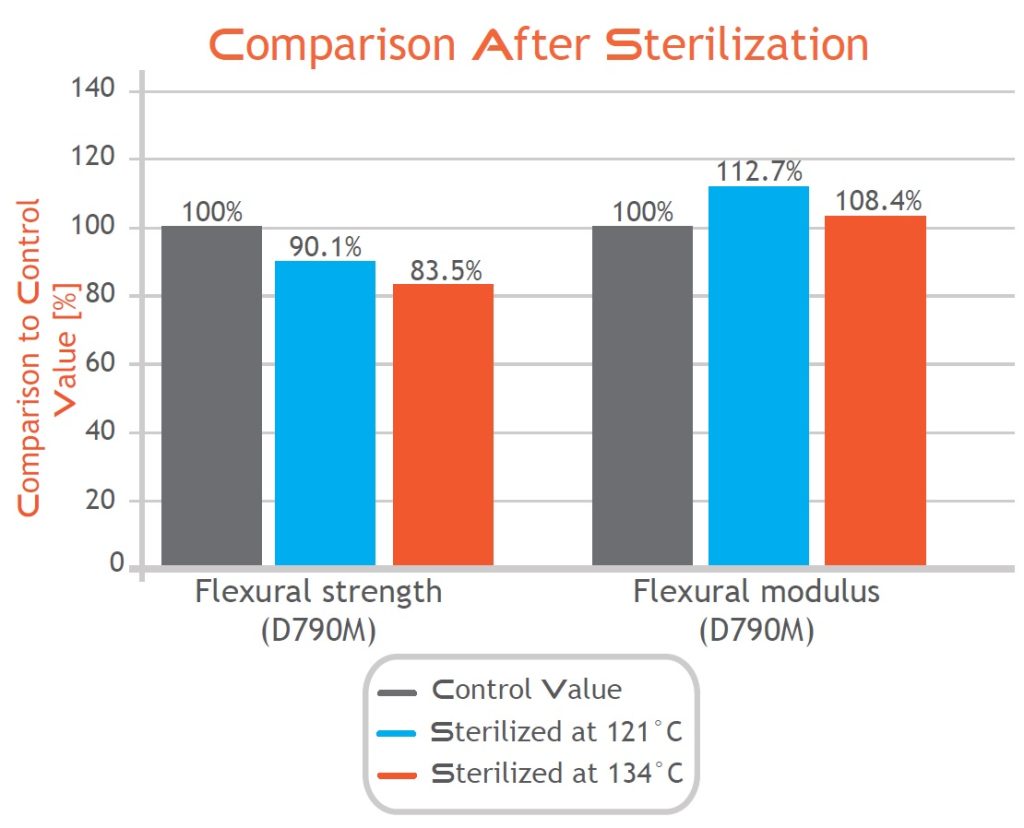
As the results in the above graph highlight, the properties of the final parts show minimal changes after steam sterilization. Due to these minimal changes, Bio-Med Clear parts can easily and safely be used in an autoclave.
OEM development for biocompatible 3D-printing resin
For OEM partners, Bio-Med Clear can be re-branded and registered for different use cases. Alongside its branded resin range, Liqcreate also provides a custom development service, offering non-standard formulas for specific applications. Through this service, customers can request the development of a polymer possessing precise characteristics, which impact its printing speed, as well as the properties of the resulting part.
As an independent resin manufacturer with R&D facilities, Liqcreate is capable of rapidly scaling its production of custom made resins where needed. Moreover, the standalone nature of the company ensures that there is minimal competition or conflict when working with 3D printer hardware manufacturers. This ensures that the firm can work quickly, and get resins to market in quantity, avoiding any issues or delays that would keep clients waiting.
Liqcreate Bio-Med Clear
Liqcreate Bio-Med Clear is a rigid clear biocompatble photopolymer resin and can be processed on most resin based 3D-printers. 3D-printed parts from this material exhibit biocompatible properties when post processed according to the processing instructions1. After washing and post-curing according to the instructions, printed parts from Liqcreate Bio-Med Clear pass the biocompatibility tests of:
| ○ Cytotoxicity | ISO 10993-5:2009 |
| ○ Sensitization | ISO 10993-10:2021 |
| ○ Irritation | ISO 10993-23:2021 |
Printed parts from Bio-Med Clear can be disinfected with commonly used disinfectants and sterilized by steam sterilization using an autoclave.
Key benefits |
3D-Printer compatibility |
| · Biocompatible | · Asiga series |
| · Steam sterilization possible | · Phrozen series |
| · High accuracy | · Elegoo & Anycubic series |
| · Dimensional stable | · And many more |
